What's the first word that comes to your mind on hearing 'mineralogy'? Minerals. Right?
How many minerals do you know about? How much do you know about these minerals?
Let’s find out more!
By the end of this topic, you are expected to;
Mineralogy is a subject in geology that specializes in the science of study of the physical properties, chemistry, and crystal structure of minerals as well as mineralized artifacts. Specific studies in mineralogy include;
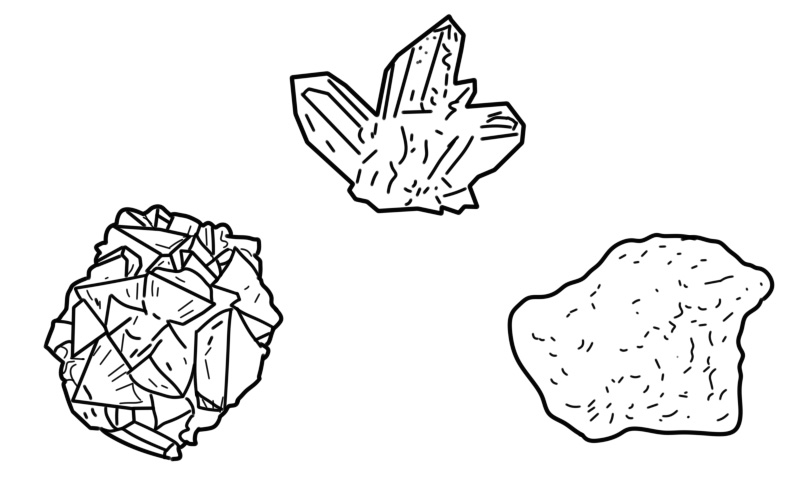
An initial step in the identification of minerals is the examination of its physical properties. Many of these properties can be measured easily on a hand sample. These properties can be grouped into density (mainly given as specific gravity), macroscopic visual properties (diaphaneity, luminescence, color, luster, streak), measures of mechanical cohesion (parting, cleavage, fracture, tenacity, hardness), and magnetic and electric properties (solubility and radioactivity in hydrogen chloride).
The hardness of a mineral is determined by being compared to other minerals. In the Mohs scale, a standard set of minerals is assigned numbers in order of increasing hardness from talc (1) to diamond (10). A harder mineral scratches a softer mineral, therefore, an unknown mineral can find its place in this scale on the basis of which minerals scratch it and those that it can scratch. A few minerals like kyanite and calcite have a hardness that is dependent on direction. Another method of measuring hardness is by measuring an absolute scale with the help of a sclerometer.
Tenacity refers to the behavior of a mineral when torn, bent, crushed, or broken. On the basis of tenacity, a mineral can be elastic, flexible, brittle, sectile, ductile or malleable. An important factor that influences the tenacity of minerals is the type of chemical bond (metallic or ionic).
Cleavage refers to the tendency to break along specific crystallographic planes.
Parting refers to the tendency of breaking along weak planes as a result of exsolution, twinning, or pressure.
Fracture is a less orderly form of breaking, which may have smooth curves that resemble the interior of a shell (conchoidal), uneven, fibrous, hackly, or splintery.
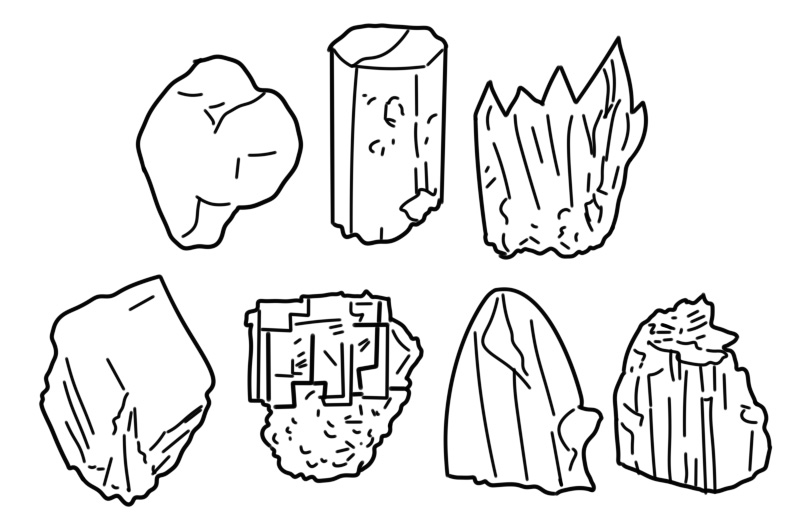
Crystal structure refers to the arrangement of atoms in a crystal. The crystal structure is represented by the use of a lattice of points repeating a basic pattern known as a unit cell, in three dimensions.
If the mineral is well crystallized, it will also have a distinctive crystal habit, for example, hexagonal and columnar that reflects the crystal structure or internal arrangement of atoms. It is also affected by crystal defects and twinning. Many crystals are polymorphic, having more than one possible crystal structure depending on factors such as pressure and temperature.
Several minerals are chemical elements such as gold, sulfur, silver, and copper but a big majority of the minerals exist as compounds. The classical method for identification of the composition is wet chemical analysis. It involves dissolving a mineral in acid like hydrochloric acid (HCl). The elements present in the solution are then identified using gravimetric analysis, volumetric analysis, or 'colorimetry'.
The environments for the formation and growth of minerals are highly varied. They range from slow crystallization at high pressures and temperatures of igneous melts deep in the crust of the earth to low-temperature precipitation from a saline brine at the surface of the earth.
Different methods of formation include;