| 1. | What is diffusion? |
| 2. | Some examples of diffusion |
| 3. | Why is diffusion useful? |
| 4. | What factors affect how materials move across the cell membrane? |
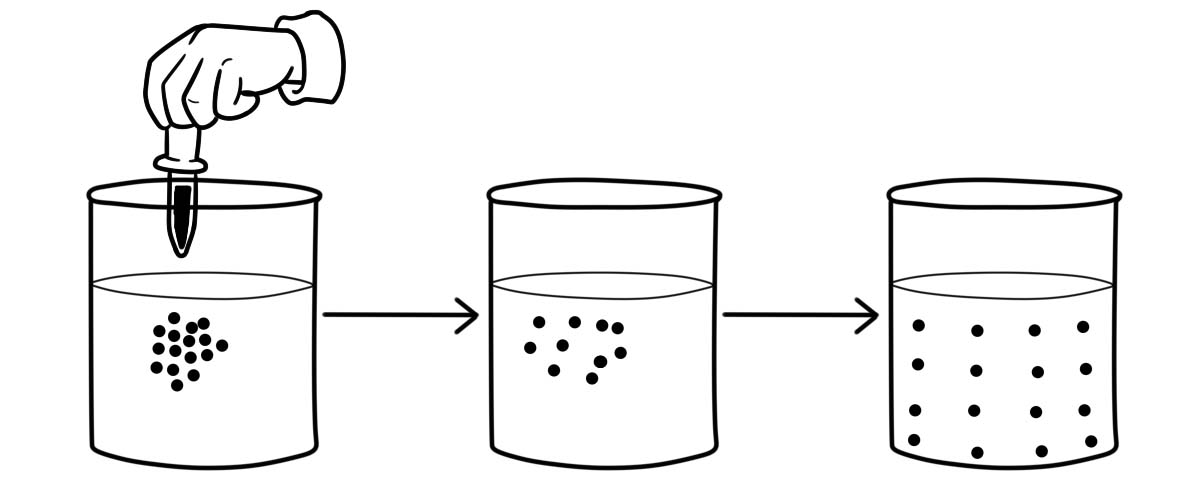
Diffusion is a physical process where molecules of material move from an area of high concentration (where there are many molecules) to an area of low concentration (where there are fewer molecules).
Diffusion usually happens in a solution of liquids and gases because their particles move randomly from place to place. It is an important process for living things; it is how substances move in and out of cells.
For example, think about someone opening a bottle of ammonia in a room filled with people. The ammonia gas is at its highest concentration in the bottle; its lowest concentration is at the edges of the room. The ammonia vapor will diffuse, or spread away, from the bottle; gradually, more and more people will smell the ammonia as it spreads.
A diffusion is a form of passive transport.
Similarly, there are more carbon dioxide molecules in the blood than in the lung so carbon dioxide molecules will tend to move into the lung. It happens in cell biology where small molecules simply diffuse through the cell membrane, but larger molecules only get through by using energy.
Some more examples of diffusion are:
In gases and liquids, particles move randomly from place to place. The particles collide with each other or with their container. The particles collide with each other or with their container. This makes them change direction. Eventually, the particles are spread through the whole container.
Diffusion happens on its own without stirring, shaking, or wafting.
In living things, substances move in and out of cells by diffusion. For example:
1. The extent of the concentration gradient – The greater the difference in concentration, the more rapid the diffusion. The closer distribution of the material gets to equilibrium, the slower the rate of diffusion becomes.
2. Mass of the molecules diffusing – Heavier molecules move more slowly, therefore, they diffuse more slowly. The reverse is true for lighter molecules.
3. Temperature – Higher temperatures increase the energy and therefore the movement of the molecules, increasing the rate of diffusion. Lower temperatures decrease the energy of the molecules, thus decreasing the rate of diffusion.
4. Solvent density – As the density of a solvent increases, the rate of diffusion decreases. The molecules slow down because they have a more difficult time getting through the denser medium. If the medium is less dense, diffusion increases.
5. Solubility: Non-polar or lipid-soluble materials pass through plasma membranes more easily than polar materials, allowing a faster rate of diffusion.
6. Surface area and thickness of the plasma membrane: Increased surface area increases the rate of diffusion, whereas a thicker membrane reduces it.
7. Distance traveled - The greater the distance that a substance must travel, the slower the rate of diffusion. This places an upper limitation on cell size. A large, spherical cell will die because nutrients or waste cannot reach or leave the center of the cell. Therefore, cells must either be small in size, as in the case of many prokaryotes, or be flattened, as with many single-celled eukaryotes.