Learning Objectives
In this lesson, we will learn
- What are enzymes?
- Main characteristics of enzymes
- How do enzymes work?
- Lock and Key Hypothesis
- Induced-Fit Hypothesis
- Key factors that affect enzyme activity
- Six different types of enzymes
WHAT ARE ENZYMES?
Enzymes are biological catalysts that speed up the chemical reactions without themselves getting altered in the process. A living system controls its activity through enzymes.
Some examples of enzymes are
- Lactase - It breaks down Lactose into Glucose and Galactose
- Catalase – It breaks hydrogen peroxide down into water and oxygen
- Glycogen synthase – It catalyzes the formation of glycosidic bonds between glucose molecules
- ATPase – It breaks down ATP into ADP, producing energy
WHAT ARE THE MAIN CHARACTERISTICS OF ENZYMES?
- The basic function of an enzyme is to increase the rate of a reaction.
- Enzymes are specific i.e. they have a specific shape, therefore only a certain substrate will fit its active site
- Enzymes are regulated from a state of low activity to high activity and vice versa
HOW DO ENZYMES WORK?
Most reactions in a cell require very high temperatures to get going, which would destroy the cell. Enzymes work by lowering the activation energy of a reaction. The activation energy of a reaction is lowered by putting stress on the bonds within a molecule, or by holding molecules close together. This increases the likelihood of a reaction and so lowers the energy required to begin it.
The molecule with which the enzyme bind is referred to as the substrate. The substrate binds to a small section of the enzyme referred to as the active site. The molecule produced at the end of the reaction is called 'product'. Once the reaction is complete, the enzyme releases the product and is ready to bind with another substrate.
There are two theories to explain the enzyme action
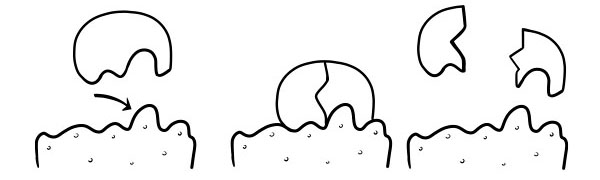
LOCK AND KEY THEORY
It was first postulated in 1894 by Emil Fischer. The Lock and Key Hypothesis is a model of how enzymes catalyst substrate reactions. It states the shape of the active sites of enzymes is exactly complementary to the shape of the substrate. When a substrate molecule collides with an enzyme whose active site shape is complementary, the substrate will fit into the active site and an enzyme-substrate complex will form. The enzyme will catalyze the reaction, and the products, together with the enzyme will form an enzyme-product complex. According to this model, it is possible for an enzyme to catalyze a reverse reaction.
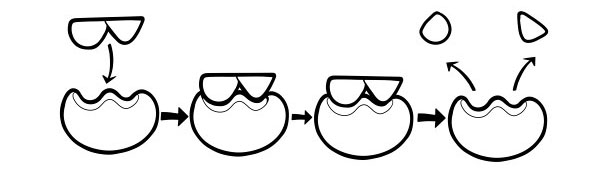
INDUCED-FIT HYPOTHESIS
This is a more recent and widely accepted model to describe the way enzymes work. It states that the shape of active sites is not exactly complementary but change shape in the presence of a specific substrate to become complementary.
When a substrate molecule collides with an enzyme, if its composition is specifically correct, the shape of the enzyme’s active site will change so that the substrate fits into its and an enzyme-substrate complex can form. The reaction is then catalyzed and an enzyme-product complex form.
FACTORS THAT AFFECT THE ENZYME ACTIVITY
The environment of the enzyme and the substrate can affect the speed of the reaction. In some cases, the environment can cause the enzyme to stop working or even unravel. When an enzyme stops working, we call it “denatured”.
Here are some factors that can affect enzyme activity:
- Temperature – The temperature can affect the reaction rate. The higher the temperature, the faster the reaction will occur. However, either increasing or decreasing the temperature outside of a tolerable range can affect chemical bonds in the active site, making them less well-suited to bind substrates. Very high temperatures may cause an enzyme to denature, losing its shape and activity.
- pH – pH can also affect enzyme function. Active site amino acid residues often have acidic or basic properties that are important for catalysis. Changes in pH can affect these residues and make it hard for substrates to bind. Enzymes work best within a certain pH range, and as with temperature, extreme pH values (acidic or basic) can make enzymes denature.
- The concentration of enzymes and substrate – The rate of reaction increases with increasing substrate concentration up to a point, beyond which any further increase in substrate concentration produces no significant change in reaction rate. This occurs because after a certain concentration of the substrate, all the active sites on the enzyme are full and no further reaction can occur.
- Inhibitors - Inhibitors are molecules that are specially made to stop the activity of enzymes. They may just slow down the reaction or stop it altogether. Some inhibitors bond with the enzyme causing it to change shape and not work correctly. The opposite of an inhibitor is an activator that can help to speed up the reaction.
TYPES OF ENZYMES
The human body consists of six major groups or classes of enzymes:
- Oxidoreductases – These enzymes enhance the rate of oxidation and reduction reactions. In these reactions, also called redox reactions, one of the reactants gives up a pair of electrons that another reactant gains. The electron-pair donor is said to be oxidized and acts as a reducing agent, while the electron-pair recipient is reduced is called the oxidizing agent. Examples include cytochrome oxidase and lactate dehydrogenase.
- Transferases – These enzymes speed along with the transfer of groups of atoms, such as methyl (CH3), acetyl (CH3CO) or amino (NH2) groups, from one molecule to another molecule. Acetate kinase and alanine deaminase are examples of transferases.
- Hydrolases – These enzymes accelerate hydrolysis reactions. Hydrolysis reactions use water (H2O) to split a bond in a molecule to create two daughter products, usually by affixing the -OH (hydroxyl group) from the water to one of the products and a single -H (hydrogen atom) to the other. In the meantime, a new molecule is formed from the atoms displaced by the -H and -OH components. The digestive enzymes lipase and sucrase are hydrolases.
- Lyases – These enzymes enhance the rate of the addition of one molecular group to a double bond or the removal of two groups from nearby atoms to create a double bond. These act like hydrolases, except that the removed component is not displaced by water or portions of water. This class of enzymes include oxalate decarboxylase and isocitrate lyase.
- Isomerases – These enzymes speed up isomerization reactions. These are reactions in which all of the original atoms in the reactant are retained, but are rearranged to form an isomer of the reactant. Isomers are molecules with the same chemical formula, but different arrangements. Examples include glucose-phosphate isomerase and alanine racemase.
- Ligases – Also called synthetases, these enzymes enhance the rate of the joining of two molecules. They usually accomplish this by making use of energy derived from the breakdown of adenosine triphosphate (ATP). Examples of ligases include acetyl-CoA synthetase and DNA ligase.

