There are three fundamental gas laws (Boyle’s Law, Charles’ Law, and Avogadro’s Law) that describes the relationship of pressure, temperature, volume and amount of gas. In this lesson, we will discuss the following
It is named after Robert Boyle.
This states that volume is inversely proportional to pressure when the temperature and the number of molecules are constant.
\(P ∝ 1/V\)
\(P = k_1V\)
where,
P is the pressure
V is the Volume
and, k1 is the proportionality constant
Now, if a fixed mass of gas undergoes an expansion at constant temperature then the final volume and pressure shall be P2 and V2.
The initial volume and initial pressure here is P1 and V1, then according to Boyle’s law:
\(P_1 \times V_1 = P_2 \times V_2 =constant (k_1) \)
\(or,\frac{P_1}{P_2} = \frac{V_2}{V_1} \)
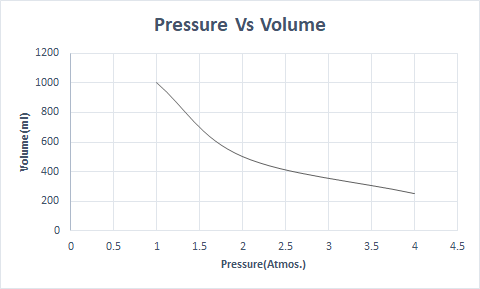
Thus, at a constant temperature, if the pressure is doubled, the volume of that gas is reduced to half. In a free state, a gaseous substance occupies a larger volume of the container due to the scattered molecules. When pressure is applied to the gaseous substance, these molecules come closer and occupy a lesser volume. In other words, the pressure applied is directly proportional to the density of the gas.
Here is the graphical representation of Boyle’s Law:
At higher altitude, atmospheric pressure is low so air is less dense. As a result, lesser oxygen is available for breathing. This is the reason mountaineers carry oxygen cylinders.
In 1787, Jacques Charles studied the effect of temperature on the volume of a gaseous substance at a constant pressure. Charles’ Law states that at constant pressure and constant mass, the volume of a gas is directly proportional to the temperature.
\(V ∝ T\)
\(V = yT\)
Where y is a constant depending on the amount of gas and pressure.
Charles’ Law is expressed as:
\(\frac{V_1}{T_1} = \frac{V_2}{T_2}\)
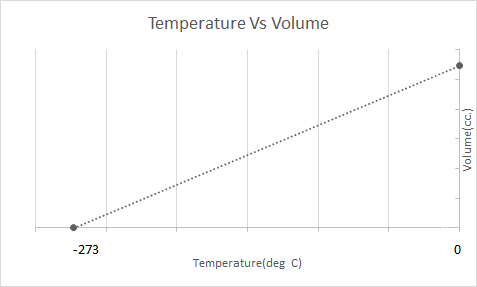
This means that with the increase in temperature the volume shall increase; with decreasing temperature, the volume decreases. In his experiment, he calculated that the volume of a given mass of a gas increases or decreases by 1/273.15 times of the original volume with every degree rise or fall in the temperature respectively.
Therefore, if the volume is V0 at 0OC and Vt is the volume at to C then,
\(V_t = V_0 + \frac{t}{273.15} V_0\)
Here is the graphical representation of the Charles’ Law:
Hot air balloons work on the basis of Charles’ Law. When a burner heats the air trapped inside the balloon, the air molecules begin to move faster and expands. The gas inside the balloon takes up more space, becoming less dense than the air surrounding it. As the hot air balloon becomes less dense, it rises up and floats.
This is also known as the Pressure Law. It was formulated by Joseph Louis Gay-Lussac in 1808.
Gay Lussac’s Law states that at constant volume, the pressure of an ideal gas is directly proportional to its absolute temperature (in Kelvin).
\(P ∝ T\)
\(P/T = k \)
where
Gay-Lussac’s Law can be expressed as:
\(\frac{P_1}{T_1} = \frac{P_2}{T_2}\)
Where:
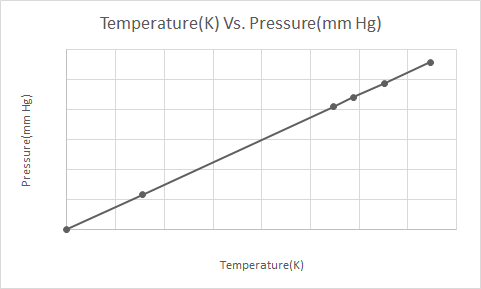
According to Gay-Lussac’s Law, the pressure of a gas (kept at constant volume) reduces constantly as it is cooled until the gas eventually undergoes condensation and becomes a liquid.
Here’s the graphical representation of the Gay-Lussac’s Law
When a pressurized aerosol can (e.g. deodorant can or spray-paint can) is heated, the resulting increase in the pressure exerted by the gases on the container can result in an explosion. This is the reason why many pressurized containers have warning labels stating that the container must be kept away from fire and stored in a cool environment.
When a pressure cooker is heated, the pressure exerted by the steam inside the container increases. The high temperature and pressure inside the container cause the food to cook faster.