As we already know, all matter on Earth exists in the form of a solid, liquid, or gas, and that solids, liquids, and gases are all made of extremely tiny particles called atoms and molecules. But all three states of matter differ one from another.
In this lesson, we are going to learn in more detail about solids. We are going to discuss the following:
Solid is one of the three fundamental states of matter. Solids are everywhere around us, the chair, the table, windows, pen, glass, jewelry, and many more. The molecules in a solid are closely packed together and contain the least amount of kinetic energy. In the solid, the particles vibrate in place. Solid volume is “the amount of space occupied by a solid object”. The volume of solid is expressed as cubic units.

Something is usually described as solid if it can hold its own shape and is hard to compress. Solids can be hard, just like cement is, or soft, like cotton; elastic like rubber, light like a plank of wood, or heavy like the lead. What is common for solids is that they have fixed shapes and fixed volumes. It is because their particles are packed closely together. This allows atoms and molecules to form chemical bonds. The particles in a solid are held together very strongly. The space between the particles is very small. The particles can vibrate, but can not move freely. That is why solids have fixed shapes and fixed volumes. So that is the reason that solids also don’t flow and are rigid, as well as they aren't readily compressible.
Solids have their own shape. They do not take the shape of their container like liquids. If you pour water (liquid) into a glass, the water will take the shape of the glass, because the water (liquids) flows. But what if you take an ice cube (we know that ice is water in solid-state) and put it into glass? The ice will not flow and take the shape of the glass. It is because of the behavior of particles. But the ice cube will soon start to melts if left at room temperature. Isn't it? This tells us that solids can change their state of matter. And they change it when they reach their melting point, by the increase of temperature. The melting point is the temperature at which a solid turns into a liquid. Different solids stay as solids at a certain temperature. The melting point of ice is 32°F (0°C). Glass, steel, copper, and diamonds are solids at room temperature. The state in which a substance exists at room temperature is called its standard state.
Let's understand this through an example. We will observe the picture below and try to distinguish the states of matter present in it.
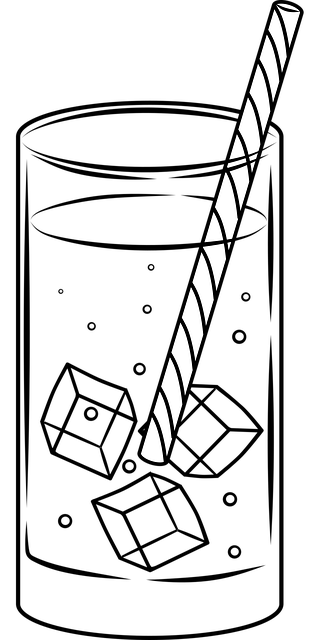
What will happen if we leave the glass for a while, at room temperature? The ice will melt, because its melting point is 0°C, and will become liquid. What about the glass? The glass will remain the same because the glass is solid at room temperature. And the plastic straw will remain solid because the plastic is solid at room temperature.
But can the melted ice become ice again? Yes, and this is done by freezing. The water from the melted ice can become ice again. So again the water will go into the solid phase.
Anything with a fixed shape and volume is an example of a solid. Examples of solids include:
Solids can be classified into two types: crystalline and amorphous.
Crystalline solids are the most common type of solid. They are characterized by a regular crystalline organization of atoms that confer a long-range order. Crystalline solids can vary in their atomic compositions, bonding, and structure. Examples include salt (sodium chloride), diamond, etc. They can be of four types:
Amorphous, or non-crystalline, solids lack this long-range order. The atoms or molecules in these types of solids are held together in a completely random formation. Examples are rubber, glass, plastic. Their use is very wide in everyday life.
Everything around us is made up of matter. So the use of solids is very wide. Our houses are made of solids (walls are made of bricks, windows are made of glass...). Our furniture too (chairs, tables, wardrobes, beds, etc). Many objects we constantly use are solids, like clothes, books, are solids. Our appliances are made of solids. The list is infinite.